One-Stop Service for Multi-Country Market Access
Relying on an experienced team of regulatory experts, Kanglife provides medical device and in vitro diagnostic medical device manufacturers with overall solutions for global market access strategies.

Oversea Marketing Strategy Counseling
With many years of experience in regulatory certification in the industry, Kanglife help manufacturers formulate the best strategies for market access in multi-countries.

Quality Management System Consulting
Relying on the team of senior regulatory experts, we provide guidance to manufacturers in establishing and upgrading quality management systems (ISO 13485, MDSAP, GMP, IVDR and MDR).

Product Testing
Kanglife has domestic testing laboratory resources and provides testing services such as safety, EMC, and chemical testing.
Pre-Clinical and Clincial Service
With abundant resources in medical laboratories and hospitals at home and abroad, Kanglife provides services such as analytical performance verification, preclinical performance verification, clinical trail and post-marketing performance evaluation.
Technical Document Consulting
Relying on the team of senior regulatory experts, Kanglife provide technical document writing, coaching and pre-review services (IVDR, MDR, NMPA, Southeast Asia, Africa, Brazil, etc.).

Local Agents
Kanglife provide third-party authorized representative services to help customers control the registered ownership of medical devices and in vitro diagnostic medical devices, and provide FSC and embassy certification services.

Customized Services
According to specific customer needs, Kanglife provide annual regulatory consulting and customized training services.
Multi-Country Market Access Service Area
Kanglife provides the customized and sustainable solution to develop the overseas markets for medical device and IVD companies, including oversea marketing strategy counseling, quality Management system counseling, product testing, performance evaluation (including analytical performance verification, preclinical verification, clinical trials, post-market performance verification, etc.), technical document counseling, and local agents , etc.provides the customized and sustainable solution to develop the overseas markets for medical device and IVD companies, including oversea marketing strategy counseling, quality Management system counseling, product testing, performance evaluation (including analytical performance verification, preclinical verification, clinical performance study, post-market performance verification, etc.), technical document counseling, and local agents , etc.
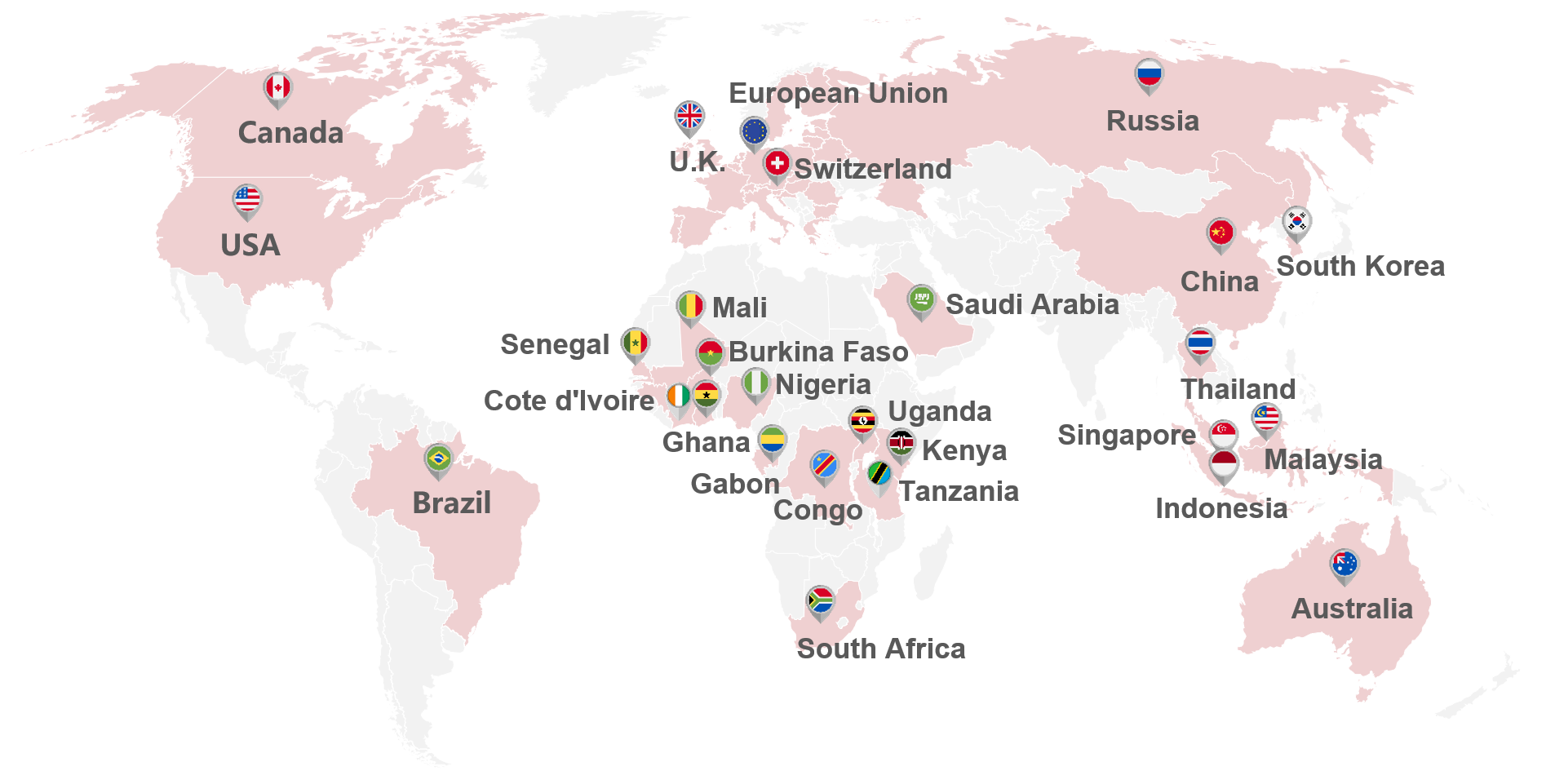
Laboratory and Clinical Resources
Kanglife, Spanish office and Thailand office have reached strategic cooperation with many laboratories and hospitals in Europe, Thailand and other regions, and can provide customers with a wealth of laboratory and clinical resources at home and abroad to meet the requirement of product testing, analytical performance verification, and preclinical performance validation, clinical trail and post-market performance evaluation, etc.
Asia

China

Thailand
Europe

Spain

Belgium

Hungary

U.K.
North America

USA
